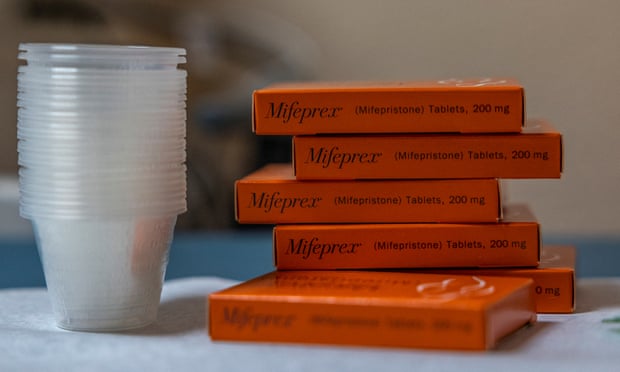
Greer Donley, associate professor at the University of Pittsburgh Law School and expert in FDA law, says the suit's legal argument is rife with "falsehoods and mischaracterizations" of the regulatory agency's decision-making. Donley says the FDA "rigorously" reviewed mifepristone before approving it, requesting additional clinical trials and restrictions in distribution. She points to a 55-page audit from the Government Accountability Office which found that FDA's approval of mifepristone was consistent with its approval and oversight for other drugs...
No comments:
Post a Comment